29+ Why Are Noble Gasses Unreactive
This means they do not need to lose or gain electrons in reactions in. Web Noble gases contain a full valence of electrons.

Synthesis Of Noble Gas Compounds Defying The Common Perception Springerlink
The noble gases are in Group 0 the farthest right on the.

. Web I want to help you achieve the grades you and I know you are capable of. Because their valency is zero. Web Answer 1 of 25.
Because of this configuration. They have a completely filled outer level. Ie their outer shell orbital of electrons is full significantly limiting their ability to form chemical bonds.
Noble gases have stable configuration due to the presence of 8 electrons in their valence shell. This means they have completely filled s and p sublevels which gives them a stable octet of. Web Why are the Noble Gases unreactive.
Because of this configuration they are i difficult to reduce electrons must enter the next valence shell. Web Advertisement Group 8A or VIIIA of the periodic table are the noble gases or inert gases. The reason as to why these elements are called noble is because.
Because of this configuration they are i difficult to reduce electrons must enter the next valence shell and ii difficult to oxidize. Web Noble gases contain a full valence of electrons. Web Explain why ionization energy decreases as you move down the noble gases from Helium to Argon.
Even if you dont want to stud. The correct option is B their valence shell is completely filled with electrons. Web elements on group 8 or 0 ie the Noble Gases all have full outermost electron shells.
Because they contain a full valence shell of electrons. Noble gases contain a full valence of electrons. Web How to succeed at GCSEs - 124 Group 0 Noble Gases.
Web 0000 - Are noble gases largely unreactive0040 - Which is the most unreactive gas0108 - What are the uses of noble gas0141 - Why is Krypton so unreacti. Compounds have been made from xenon and krypton but not from other noble. Web Noble gases are six monoatomic gaseous elements found in nature that share similar chemical properties.
One of the defining properties of this group is the unreactivity of these elements. Helium He neon Ne argon Ar krypton Kr xenon Xe and radon Rn. These grades are the stepping stone to your future.
Web Why noble gases are so unreactive. The atoms of noble gases already have complete outer shells so they have no tendency to lose gain or share electrons. A chemical bond is.
Web Why are noble gases relatively unreactive. Web The elements that make up the last group in the periodic table are called noble gases. Web Answer and Explanation.
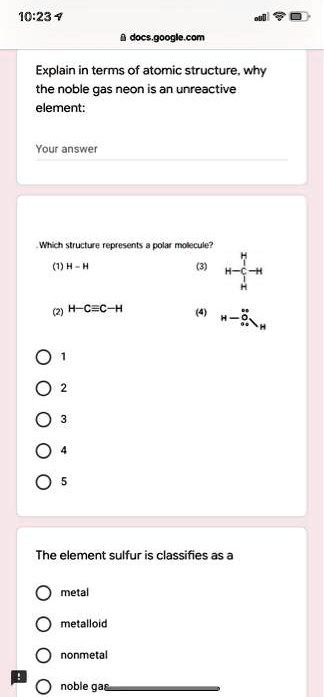
Solved 10 23 Docs Google Com Explain In Terms Of Atomic Structure Why The Noble Gas Neon Is An Unreactive Element Your Answer Witc Structure Represents Pola Motecule H 2 H C C H 4 3
Noble Gases Uncyclopedia The Content Free Encyclopedia

Stable Octets Why Are The Noble Gases Unreactive Ppt Download
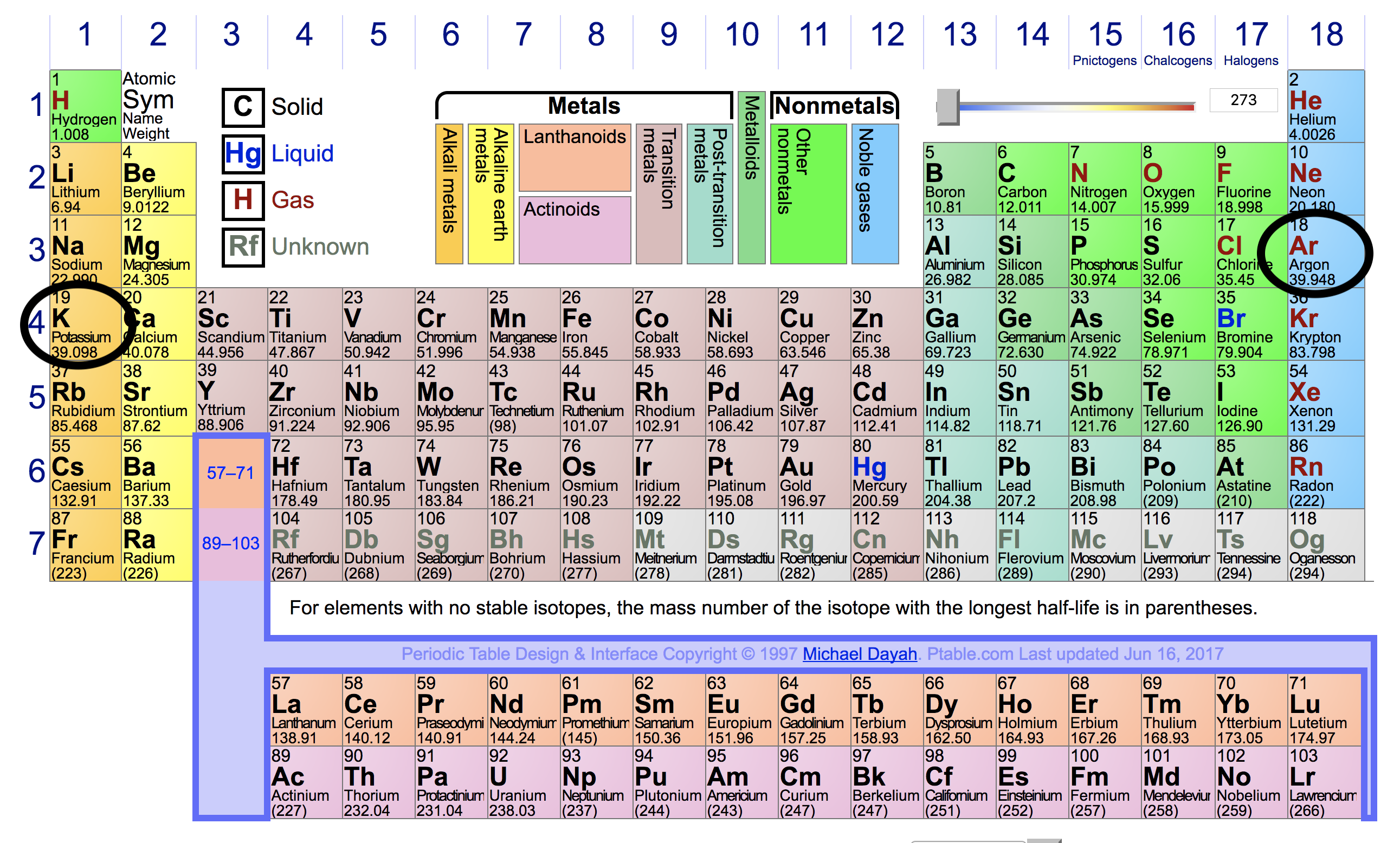
Potassium Is Highly Reactive Metal While Argon Is An Inert Gas How Can You Explain This Difference Based On Their Electron Configurations Socratic
Synthesis Of Noble Gas Compounds Defying The Common Perception Springerlink

Noble Gases 8 2 5 Cie Igcse Chemistry Revision Notes 2023 Save My Exams

Periodic Table Song Noble Gases Noble Gases Noble Gases Song Youtube

Magnetochemistry Free Full Text Explorations Of Magnetic Properties Of Noble Gases The Past Present And Future

Difference Between Inert Gases And Noble Gases Knowswhy Com

Solved Question 3 Noble Gases Are Unreactive Because O They Chegg Com

Impossible Chemistry Forcing Noble Gases To Work New Scientist
Noble Gas Hi Res Stock Photography And Images Alamy

Noble Gases Trends And Patterns Scienceaid

Noble Gases Definition

Noble Gases Ck 12 Foundation
Impossible Chemistry Making The Unreactive React

Why Are Noble Gases Unreactive Youtube